Science
Advancing innovative therapies for neuropsychiatric disorders
Scientific Foundations of Our Approach
At Praxis Bioresearch, our discovery platform is built on understanding the fundamental neurobiology of CNS disorders and translating these insights into innovative therapeutic solutions. Our lead candidate, PRX-P4-003, exemplifies this approach through its novel, clinically validated gut-specific prodrug mechanism, designed to address significant unmet medical needs.
- Full In-House Development: Our compound was discovered and developed entirely in-house from initial discovery through clinical stage, providing complete IP ownership, deep scientific insight, and full strategic control over development pathways. This integrated approach ensures exceptional quality control and creates strategic advantages for all stakeholders through comprehensive knowledge transfer, regulatory documentation, and seamless development continuity.
PRX-P4-003 is a selectively bioavailable prodrug of fencamfamine isomer, a well established dopamine norepinephrine reuptake inhibitor with distinct advantages over current treatments.
Pure reuptake inhibition at DAT
Combined reuptake inhibition and mild ability to release dopamine. Simplified visualization based on available scientific data and expert interpretation.
Multiple release mechanisms including DAT reversal and VMAT2 disruption
While both fencamfamine and methylphenidate act as reuptake inhibitors at the dopamine transporter (DAT), fencamfamine's additional dopamine-releasing activity (though ~10-fold less potent than amphetamine) distinguishes it from methylphenidate's "pure" reuptake inhibition*. Although the focus in these visualizations is on dopamine signaling, it is important to note that these compounds modulate norepinephrine through analogous mechanisms, contributing to their therapeutic effects1.
Amphetamine can trigger rapid, intense dopamine release through multiple mechanisms, leading to high efficacy but increased risk of side effects and abuse potential2,3,4,5,6. Meta-analyses of ADHD studies indicates that methylphenidate while effective may have lower overall efficacy compared to amphetamine (SMD -0.78 vs -1.02)7,8. Amphetamine is banned in China, Japan, and many countries in Europe and Middle East likely due to concerns about amphetamine's abuse potential and dependency risk. In these countries where amphetamines are restricted, alternative treatments like methylphenidate or non-stimulant medications are often used for ADHD management.
ATTENTION DEFICIT HYPERACTIVITY DISORDER (ADHD)
A NOVEL APPROACH TO ADHD TREATMENT
ADHD affects approximately 4.4% of the population worldwide, with more adults today than children receiving diagnosis11. While current stimulant medications are effective, they present significant challenges that PRX-P4-003 aims to address:
PRX-P4-003 offers multiple strategic advantages: potential Schedule IV classification enhancing accessibility and compliance; balanced pharmacology combining reuptake inhibition with mild dopamine release for optimal efficacy and safety; built-in abuse deterrence through gut-specific activation that renders the compound biologically inactive when misused; natural long-acting profile (~15 hours) based on fencamfamine's inherent sustained action providing optimal pharmacokinetics without complex modification; and established safety profile leveraging fencamfamine's proven clinical history in related stimulant indications9,10,11,12.
APATHY IN ALZHEIMER'S DISEASE
CURRENTLY, THERE IS NO FDA-approved therapy for apathy in Alzheimer's disease
Apathy is the most common noncognitive symptom in Alzheimer's Disease, affecting nearly 70% of the patients. Untreated apathy can lead to poor quality of life for the patient, rapid decline in cognition, and increased caregiver distress, and may result in costly early institutionalization.
Recent clinical research demonstrates that apathy is responsive to a dopaminergic approach13,14,15. As the first potential FDA-approved treatment specifically targeting apathy in AD, our approach could improve efficacy, enhance patient outcomes and daily functioning, while simultaneously reducing caregiver burden and associated costs. This therapy may deliver substantial healthcare system impact through delayed institutionalization and addressing a significant component of the global dementia burden.
A GUT-SPECIFIC PRODRUG SOLUTION
Our lead development candidate, PRX-P4-003, is a stearic acid conjugate of an active isomer of fencamfamine [(-)-FCF], created to provide an innovative, low-risk prodrug16,17. Fencamfamine, a well-tolerated Schedule IV stimulant, has an established 5 decades of therapeutic use history.
PRX-P4-003: New Chemical Entity (NCE)
IUPAC/CAS: Octadecanoic acid, [[[ethyl[(1R,2R,3S,4S)-3-phenylbicyclo[2.2.1]hept-2-yl]amino]carbonyl]oxy]methyl ester
- "Composition of Matter" patents in multiple countries (US/Europe/China/Japan) with anticipated protection to 2042
- IND cleared by FDA for Phase 1 studies
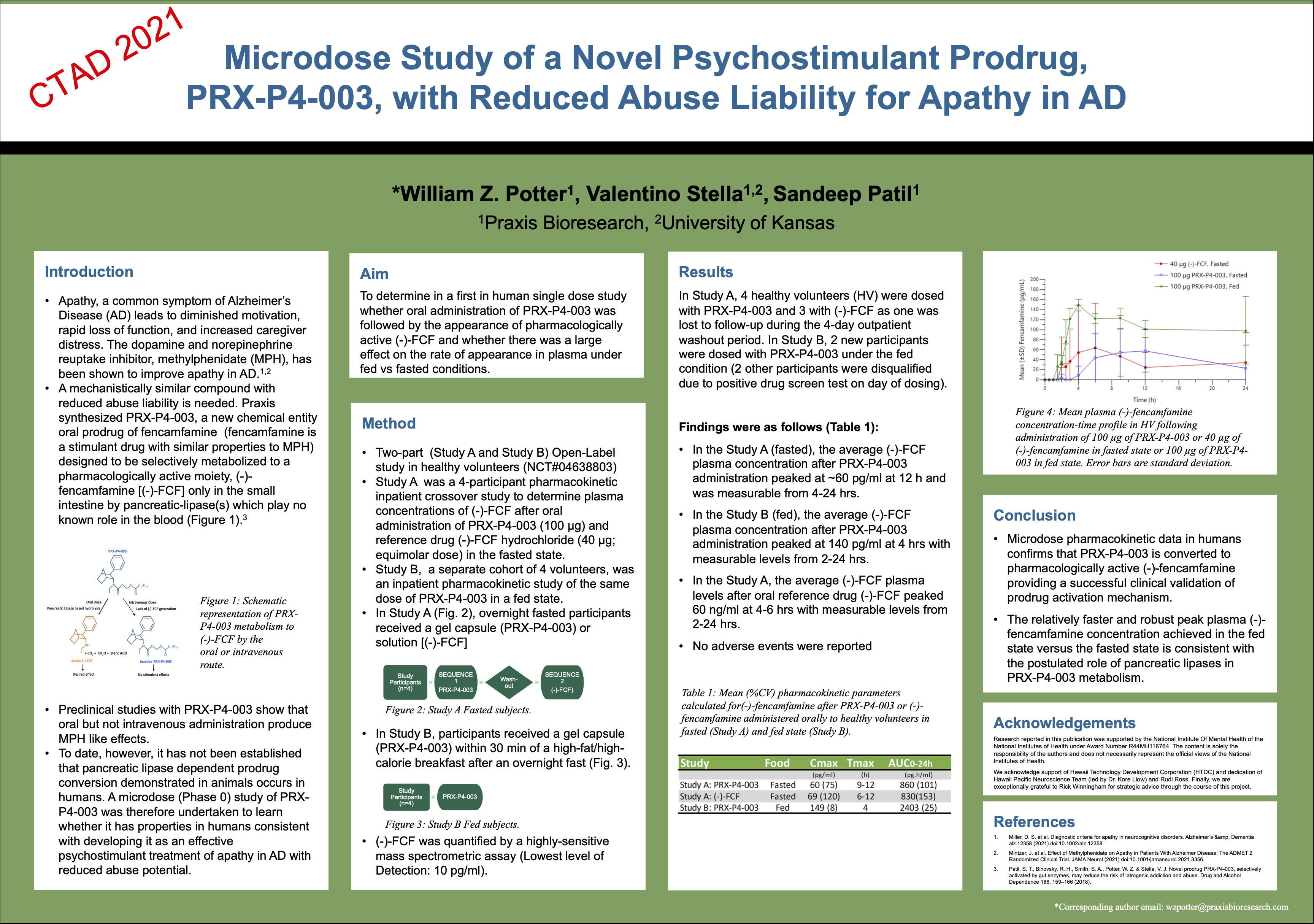
- Successful microdose PK study in humans
- GMP drug product already manufactured and available for clinical trials
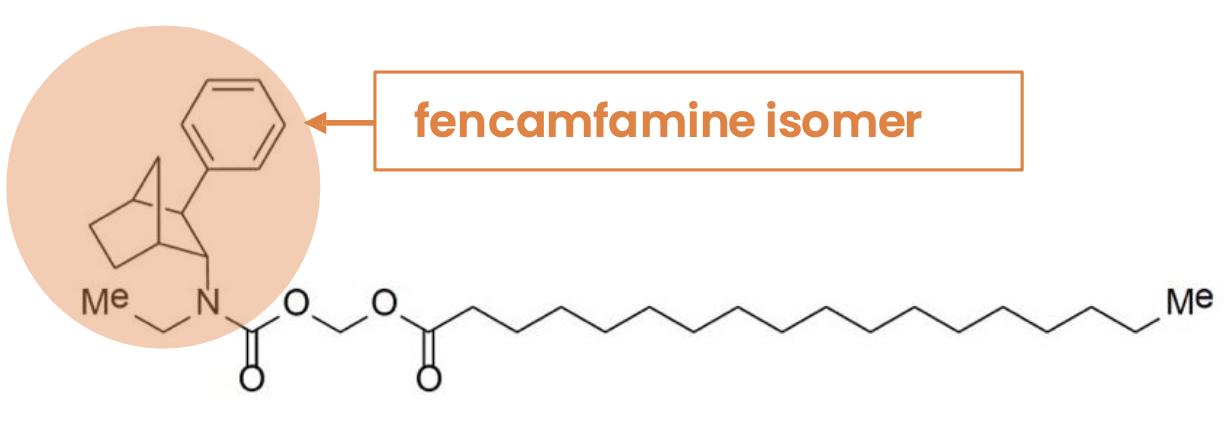
PRX-P4-003 is designed to be activated by pancreatic lipase, an enzyme whose biological activity is mainly in the gut. Less than 1% of pancreatic lipase is active in the intravenous compartment. This activation mechanism not only facilitates its therapeutic action but also serves as a safeguard against misuse.
PRX-P4-003 is biologically inactive and has to be enzymatically cleaved in the gut to release the parent drug (-)-FCF (a purified fencamfamine isomer), which is responsible for its stimulant-like effects. This novel mechanism has been proven in human studies, with demonstrated conversion to the active compound in plasma (see poster presented below at 14th Conference Clinical Trials on Alzheimer's Disease, November 2021).
* PRX-P4-003 is designed to deliver (-)-fencamfamine (DAT Ki 0.07 µM versus methylphenidate DAT Ki 0.06 µM), the active isomer comprising 45% of marketed racemic fencamfamine. CEREP studies confirm DAT/NET inhibition and absence of MAO inhibition, with preclinical findings matching the established profile of racemic fencamfamine. While vesicular dopamine release for (-)-fencamfamine has not been specifically tested in vitro, it is expected to parallel the racemate's activity
References
- Han DD, Gu HH. Biochem Pharmacol. 2006;72(2):235-241
- Volkow ND, et al. Am J Psychiatry. 2002;159(11):1909-1918
- Heal DJ, et al. Neuropharmacology. 2013;87:41-54
- McMillen BA. CNS stimulants: two distinct mechanisms of action for amphetamine-like drugs. TIPS Review 1983;4:429-432
- Reith MEA, Gnegy ME. Molecular Mechanisms of Amphetamines. In: Substance Use Disorders. 2019:265-297
- Sulzer D, et al. Neuropharmacology. 2005;47:S47-S53
- Cortese S, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5(9):727-738
- Faraone SV, et al. The World Federation of ADHD International Consensus Statement. Neurosci Biobehav Rev. 2021;128:789-818
- Holliday, A. R.; Devery, W. J. Clin. Pharmacol. Ther. 1962, 3, 5
- DeLucia, R.; Planeta, C. S. Gen. Pharmacol. 1990, 21 (2), 161
- Seyfried CA. Dopamine uptake inhibiting versus dopamine releasing properties of fencamfamine: An in vitro study. Biochem Pharmacol. 1983;32(15):2329-2331
- Faraone SV. J Clin Psychiatry. 2018;79(5):18r12458
- Mintzer, J. ADMET Investigators. JAMA Neurol (2021) doi:10.1001/jamaneurol.2021.3356
- Rosenberg et al. ADMET Investigators. J Clin Psychiatry 2013, 74, 810
- Padala et al. Am J Geriatr Psychiatry 2010, 18 (4), 371
- Patil ST, et al. Novel prodrug PRX-P4-003, selectively activated by gut enzymes, may reduce the risk of iatrogenic addiction and abuse. Drug Alcohol Depend. 2018;186:159-166
- Patil, S., Bihovsky, R., Smith, S., Li, Y., Stella, V., Long, D.D., Marques, D., Altman, D.E., inventors; Praxis Bioresearch, LLC, assignee. Prodrugs of fencamfamine. US Patent Application WO/2017/048720. 2016
